ECAT 2017 CHEMISTRY PORTION
- What is the empirical formula for glucose?
- Which of the following statements is correct?
- Base principle of crystallization is:
- The bubbling up of gas from soda drink is best explained by:
- The effect of pressure on density of gas is explained as under:
- Forces which make the liquefaction of Helium gas possible are:
- Existence of sulphur in two forms is:
- The low boiling point of hydrofluoric acid (HF) as compared to water (H2O) is due to:
- Radiations emitted in the form of photons when electrons of Hydrogen atom fall from higher level to n = 1 are in the:
- The structure of Nitrogen molecule (N2) is explained by:
- The solubility of sodium chloride in water is possible because:
- Calculate enthalpy change in formation of NaHCO3 (aq) using Hess’s law:
- According to the first law of thermodynamics, if thermal energy is applied to water placed in a cylinder fitted with a friction-less piston:
- NaCl is not soluble in acetone because:
- Ethylene glycol is mixed with water in automobile radiators as antifreeze because:
- Oxidation number of sulphur in is:
- During the purification process of copper, a thin sheet of pure and impure copper is placed in electrolytic cell, which results in:
- The purpose of two half cells in a galvanic cell is:
- Catalyst helps in a reaction by:
- Sub-group “B” of the periodic table represents:
- When an electron is added to O then the energy change is expressed by:
- The important usage of lime in agriculture is:
- Quartz crystal has typical tetrahedral structure between oxygen and silicon atoms. How many silicon atoms are connected to an oxygen atom in this structure?
- In the reaction
- In the reaction
- True statement(s) about para-magnetic property of transition elements is/are:
- Tubes made of steel can be hammered while hot, but a cutting tool also made of steel cannot be hammered while hot. This due to:
- Alkanes are less reactive as compared to alkenes because:
- Important property of polymers of PVC pipes is:
ANSWERS
For any info, please write in comment















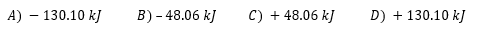

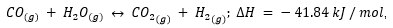





















0 Comments